The Glenmark Pharmaceuticals and American Health Packaging have recalled 141 and 21 batches, respectively, of their Potassium Chloride Extended-Release Capsules (750 mg, 10 mEq K). Read further.
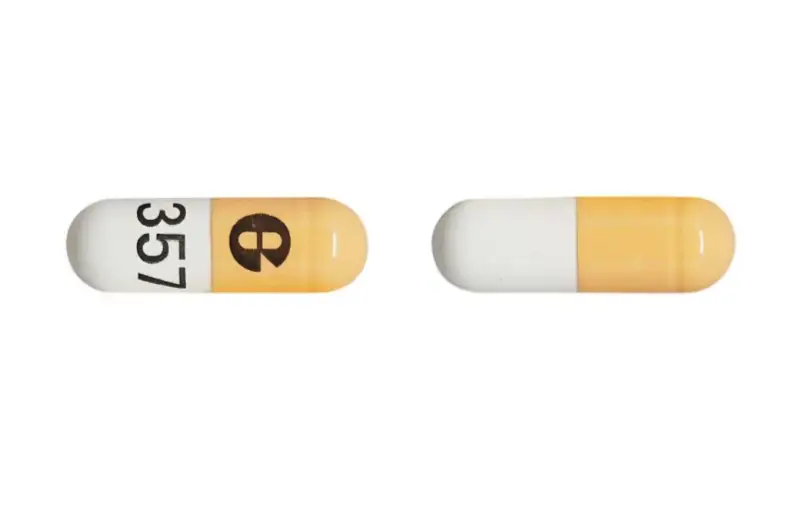
What To Know about the Recall
Glenmark Pharmaceuticals recalled 114 batches, and American Health Packaging recalled 21 batches of Potassium Chloride Extended-Release Capsules (750 mg, 10 mEq K) sold directly to consumers. “The failed dissolution of potassium chloride extended release capsules may cause high potassium levels, also known as hyperkalemia, which can result in irregular heart beat that can lead to cardiac arrest”.
Recall of Blood Pressure Medication
Two recalls were issued last month for potassium chloride extended-release capsules used to control blood pressure due to concerns about dissolution problems.
Treatment for Recalled Medication
The recalled medication treats low potassium and has been distributed nationwide to wholesalers, distributors, and retailers.
Medical Advice on Recalled Medication for Consumers
People who use the recalled medication should talk to their doctor before stopping it and inform them of any issues they experience that might be related to the medication.
Wholesalers, distributors, and retailers should stop distributing the recalled medication right away and arrange for a recall at the retail or pharmacy level.
READ —